ENCE 433 Dr.
Alba Torrents
ENVIRONMENTAL ENGINEERING
ANALYSIS Spring 2001
Homework Set #
1 Out:
February 1 Due:
February 8
SOLUTIONS
1.
Molality is grams of solute per
kilogram of water.
![]()
2.
(a) The molecular weight of CO3-2
is 60.
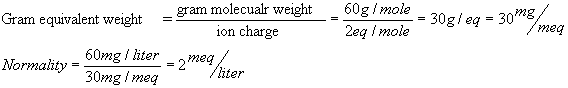
(b) The molecular weight of Ca3(PO4)2
is 310. Because each Ca3(PO4)2 forms six positive and six negative charges,
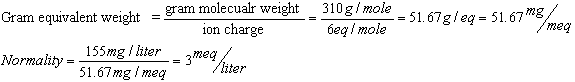
3.
Mol. wt. CaSO4 = 136
Mol. wt. Na2CO3 = 106
This is really a titration problem. We don't worry about normalities here because the stoichiometric
coefficients of the reactants are equal.
The number of moles of
CaSO4 to be softened
is:
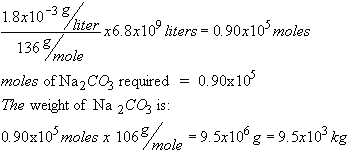
About 10 tons.